Chemistry
The matchstick puzzle fun
The higher order pyrrole-imidazole alkaloids are among the most synthetically challenging natural products, and their biosynthesis is poorly understood. Following the hypothesis that marine sponges use single-electron transfer (SET) as a central mechanism to promote [2+2], [3+2] and [4+2] cycloadditions, we have designed a single-electron oxidation reaction to mimic this biogenic dimerization process. Our computational and experimental studies suggest that sceptrin and ageliferin may arise from [2+2] and [4+2] homodimerization of two hymenidin molecules, respectively. By contrast, massadine may be assembled from one oroidin and one dispacamide A molecule through [3+2] heterodimerization. The pathway selectivity is likely determined by the relative stability of the radical intermediates. In addition to using this strategy to complete the asymmetric synthesis of sceptrin, ageliferin, massadine and axinellamine, we have also revised the absolute stereochemistry of the [2+2] and [4+2] dimers. Surprisingly, the [2+2] and [4+2] dimers are antipodal to the [3+2] dimers. Such stereochemical relationship suggests that an enantiodivergent pathway is involved in the biosynthesis of these alkaloids. Another class of structurally unique and biologically interesting natural products is the C-nor-D-homosteroids. Nakiterpiosin and nakiterpiosinone are the only known C-nor-D-homosteroids of a marine origin. Unlike their terrestrial congeners, these molecules possess a unique skeleton and are heavily halogenated. We have developed a convergent approach to accomplish their total synthesis, revised their relative stereochemistry, and provided insights to their biological functions.
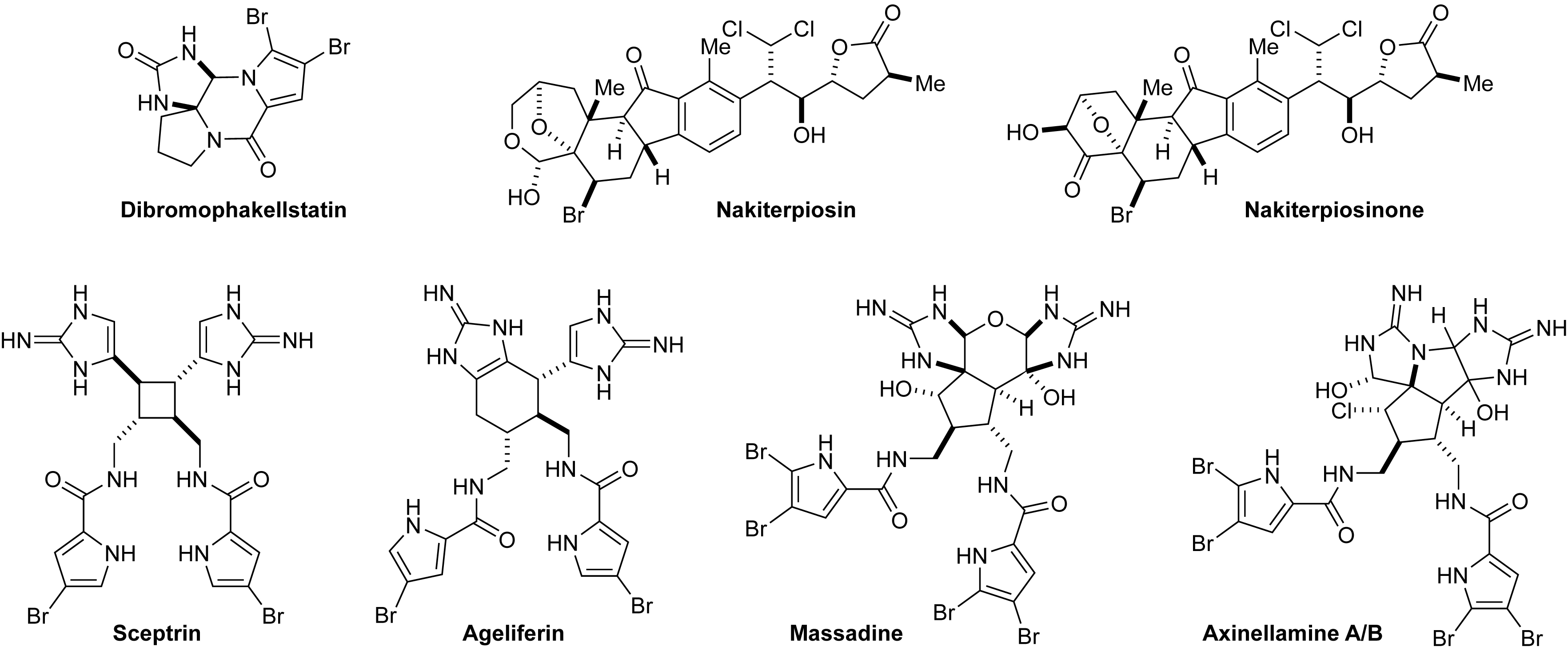
Palladium-Catalyzed Direct Functionalization of Imidazolinone: Synthesis of Dibromophakellstatin
Lu, J.; Tan, X.; Chen, C.
J. Am. Chem. Soc. 2007, 129, 7768–7769 (PMID: 17539649)
Asymmetric Synthesis of Ageliferin
Wang, X.; Ma, Z.; Lu, J.; Tan, X.; Chen, C.
J. Am. Chem. Soc. 2011, 133, 15350–15353 (PMID: 21888421) (correction)
Asymmetric Syntheses of Sceptrin and Massadine and Evidence for Biosynthetic Enantiodivergence
Ma, Z.; Wang, X.-L.; Wang, X.; Rodriguez, R. A.; Moore, C. E.; Gao, S.; Tan, X.; Ma, Y.; Rheingold, A. L.; Baran, P. S.; Chen, C.
Science 2014, 346, 219–224 (PMID: 25301624)
Syntheses of Sceptrins and Nakamuric Acid and Insights into the Biosyntheses of Pyrrole–Imidazole Dimers
Wang, X.-L.; Gao, Y.; Ma, Z.; Rodriguez, R. A.; Yu, Z.-X.; Chen, C.
Org. Chem. Front. 2015, 2, 978–984 (PMID: 26328059)
Asymmetric Synthesis of Axinellamines A and B
Ma, Z.; Wang, X.; Ma, Y.; Chen, C.
Angew. Chem. Int. Ed. 2016, 55, 4763–4766 (PMID: 27037993)
Synthesis and Structure Revision of Nakiterpiosin
Gao, S.; Wang, Q.; Chen, C.
J. Am. Chem. Soc. 2009, 131, 1410–1412 (PMID: 18998640)
Chemical and Biological Studies of Nakiterpiosin and Nakiterpiosinone
Gao, S.; Wang, Q.; Huang, L. J.; Lum, L.; Chen, C.
J. Am. Chem. Soc. 2010, 132, 371–383 (PMID: 20000429)
New brushes for molecule painting
Metalloenzyme-catalyzed C–H and olefin oxidations are heavily involved in the biosynthesis and metabolism of small molecules. We have developed various vanadium catalysts to broaden the scope of metal-catalyzed C–H functionalization chemistry. During this process, we found that photoexcited carbon-oxo (carbonyl) species are functionally similar to the metal-oxo complexes, which inspired us to develop new C–H functionalization reactions that do not require the use of transition metals. We have further found that these photochemical reactions can be promoted by off-band irradiation at wavelengths significantly longer than generally believed (>50 nm away from λmax). In particular, visible light generated from a household compact fluorescent lamp can drive photoreactions of various colorless monoaryl ketones and eneons.
A Highly Selective Vanadium Catalyst for Benzylic C–H Oxidation
Xia, J.; Cormier, K. W.; Chen, C.
Chem. Sci. 2012, 3, 2240–2245 (PMID: 22712051)
Vanadium-Catalyzed Oxidative Strecker Reaction: α-C–H Cyanation of para-Methoxyphenyl (PMP)-Protected Primary Amines
Zhu, C.; Xia, J.-B.; Chen, C.
Tetrahedron Lett. 2014, 55, 232–234 (PMID: 24415804)
Vanadium-Catalyzed C(sp3)–H Fluorination Reactions
Xia, J.-B.; Ma, Y.; Chen, C.
Org. Chem. Front. 2014, 1, 468–472 (P)MID: 24976971)
Visible Light-Promoted Metal-Free C–H Activation: Diarylketone-Catalyzed Selective Benzylic Mono- and Difluorination
Xia, J.-B.; Zhu, C.; Chen, C.
J. Am. Chem. Soc. 2013, 135, 17494–17500 (PMID: 24180320)
Visible Light-Promoted Metal-Free sp3-C–H Fluorination
Xia, J.-B.; Zhu, C.; Chen, C.
Chem. Commun. 2014, 50, 11701–11704 (PMID: 25143256)